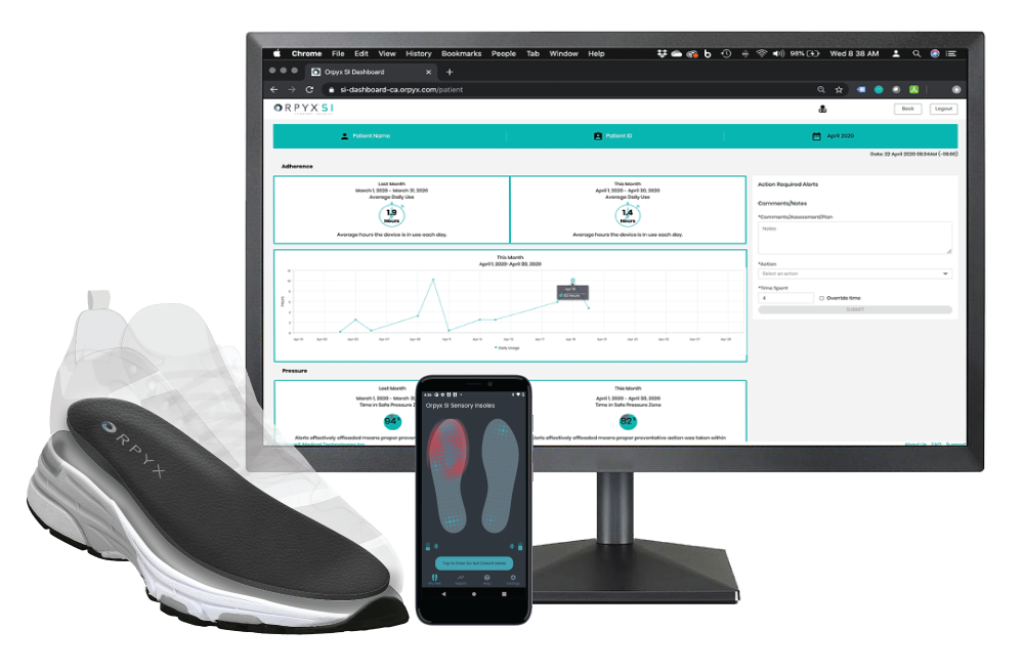
For contract research organizations
Simplify data collection and ease patient burden

Majority of RCTs miss recruitment targets or experience delays¹
Traditional clinical studies are designed around capabilities and availability of trial sites often leading to challenges in protocol complexity, slow enrollment, low patient retention and limited geographic reach of patients, all of which extend trial timelines and increase costs.2
Boost data quality, quantity, and collection frequency³
Digital health tech with remote patient monitoring can:
- Lower costs, shorten timelines, limit patient fatigue and increase retention by reducing the frequency of onsite patient visits4
- Improve recruitment, diversity, reach, and retention through remote care by broadening the patient geographical pool4, 5, 6
- Allow patients to be monitored effectively and enable physicians to intervene based on real-time data3
- Be less reliant on participant-reported information to generate more objective data that links clinical research to real-world data activities7
- Ongoing monitoring can produce more granular information leading to early detection of adverse outcomes, and in turn, earlier identification of target therapy efficacy2

Diabetes healthspan extension™
The Orpyx platform has the potential for broad future use in the areas of activity quantification and qualification, gait and balance assessment, weight change detection, and general health status analysis. Future care pathway expansion includes chronic disease management, perioperative care, and vascular applications. Advancements in applied AI and ML will allow for continuous evolution of insights derived from the analysis of collected data.
Orpyx is focused on collaborating with stakeholders to achieve a healthcare future that improves patients' quality of life while improving overall clinical outcomes and mitigating costs. We look forward to further discussions around ways we can partner to achieve your trial goals.
A comprehensive turn-key solution for RPM
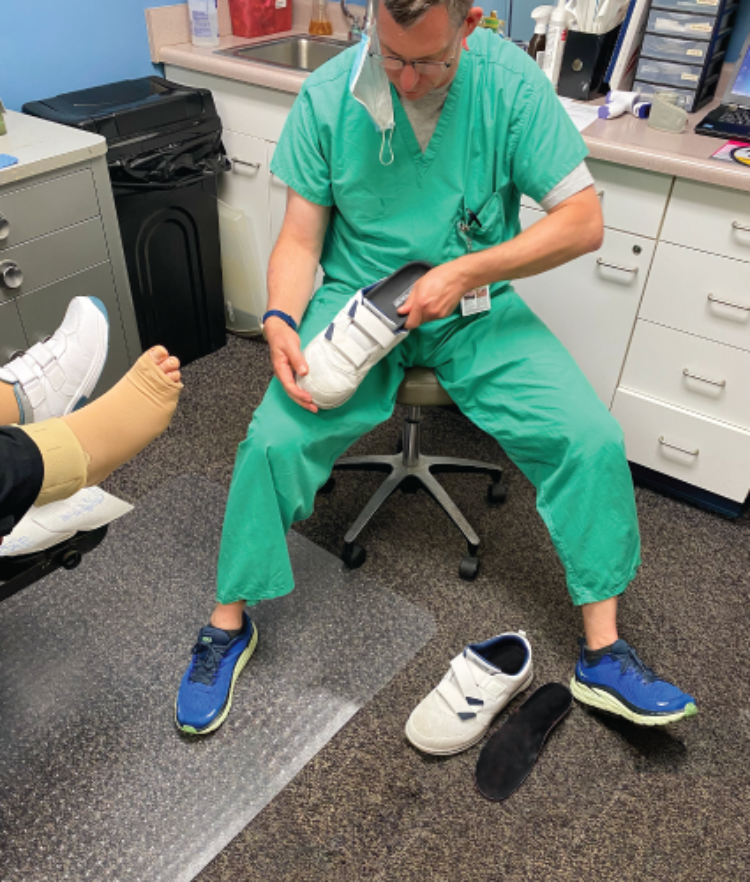
The Orpyx SI® Sensory Insole system with remote patient monitoring services helps monitors and manage participants through real-world data collection.
- The only system that provides real-world plantar pressure data backed by an RCT. Our study show patients experienced fewer ulcers (relative risk reduction of 86%)9
- Enables clinicians to observe and assess data in near real-time, and adjust care plans as needed
- Automatically and continuously collects real-world data reducing study team burden and participant fatigue
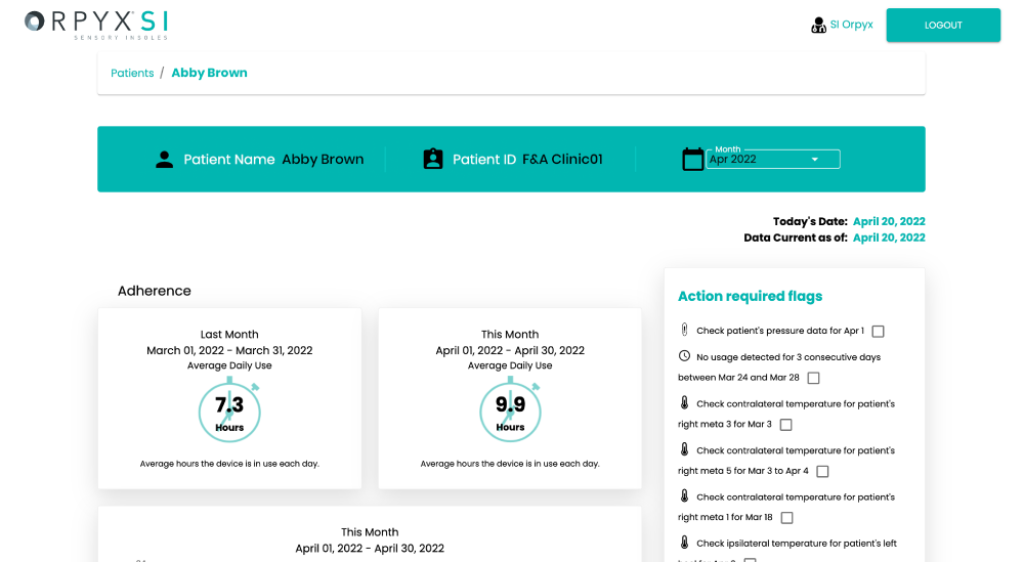
- Provides actionable data and updates through the Dashboard to monitor utilization and adherence
- Patient engagement encouraged but not required via the Orpyx SI app
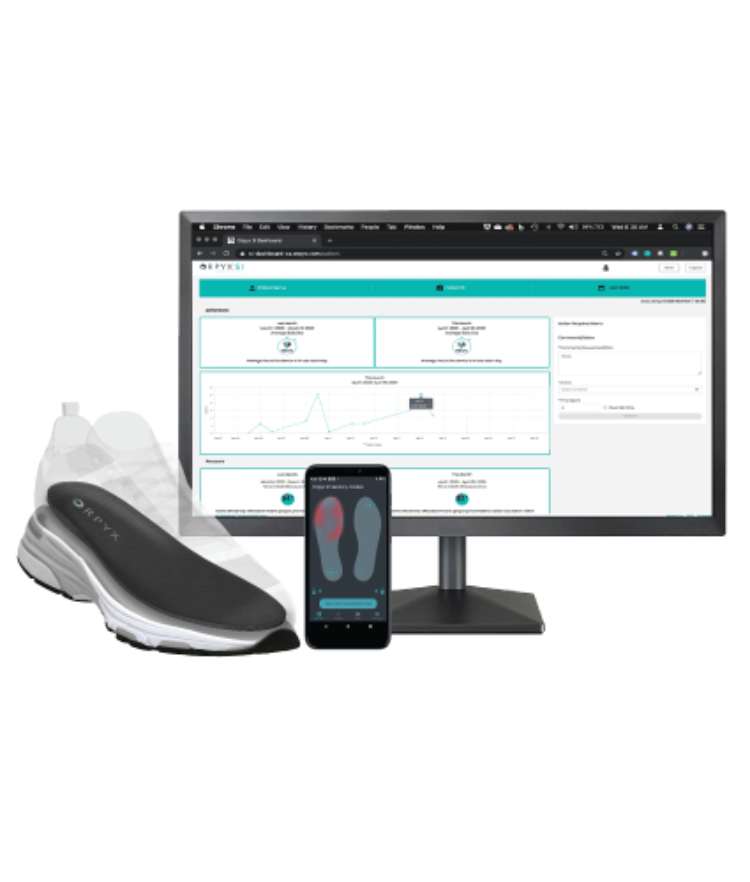
Simple dashboard access and reporting capabilities
- Cloud-based, HIPAA-compliant dashboard for on-demand data access by credentialed remote patient monitors
- Data is refreshed daily enabling ability to monitor protocol adherence and product utilization
- Data is easily exported
- Ease of data collection for CRO, study teams, and participants
1. B.G.O. Sully, S.A. Julious, J. Nicholl. A reinvestigation of recruitment to randomised, controlled, multicenter trials: a review of trials funded by two UK funding agencies; Trials, 14 (2013), p. 166 2. Personalising Digital Health: How to develop and deploy novel technologies to reduce patient burden and increase engagement [White paper]. ICON (2019) 3. McCarthy M, Ballinger R, Lewis H. Advancing digital endpoints: An end-to-end approach to managing wearable devices through clinical development [White paper]. ICON (2020) 4. Sommer C, et al. (2018) Building clinical trials around patients: Evolution and comparison of decentralized and conventional site models in patients with low back pain. Contemporary Clinical Trials Communications 11:120-126 5. ‘No Place like Home? Stepping up the Decentralization of Clinical Trials.’ McKinsey & Company, 11 June 2021; https://www.mckinsey.com/indus... 6. Goodson N, et al (2022). Opportunities and counterintuitive challenges for decentralized clinical trials to broaden participant inclusion. Npj Digital Medicine, 5, 58. https://doi.org/10.1038/s41746... 7. Fantana T, Combs A, & Li J. (2022, September 16). How Remote Patient Monitoring Can Impact Decentralized Clinical Trials. Applied Clinical Trials. https://www.appliedclinicaltri...patient-monitoring-technology-can-impact-decentralized-clinical-trials 8. Najafi, B., et al (2018). Cost effectiveness of smart insoles in preventing ulcer recurrence for people in diabetic foot remission. Wound Care Management, 7, 1-7. https://doi.org/10.15761/WCM.1... 9. Abbott CA et al. Innovative intelligent insole system reduces diabetic foot ulcer recurrence at plantar sites: a prospective, randomised, proof-of-concept study. The Lancet Digital Health. Vol.1 October 2019 * Study was conducted with irst generation SurroSense Rx® which has the same pressure algorithm as Orpyx SI Sensory Insoles